AD Pipeline Watch: U.S. FDA Clears IND for Zabalafin Hydrogel in AD
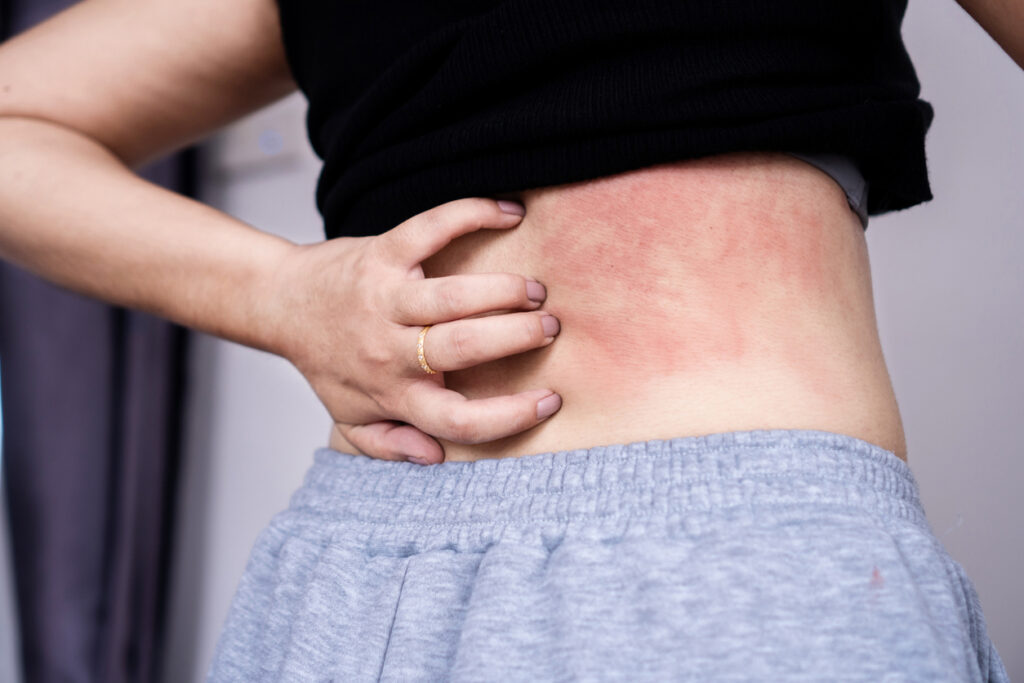
The U.S. Food and Drug Administration (FDA) has cleared the Investigational New Drug Application (IND) for Alphyn’s Zabalafin Hydrogel for the treatment of mild-to-moderate atopic dermatitis (AD). Zabalafin Hydrogel is a novel, first-in-class complex single-source botanical drug with multiple bioactive compounds that provide multiple mechanisms of action, including anti-pruritic, antibacterial, and anti-inflammatory activity. Two Phase […]
