AD Pipeline Watch: U.S. FDA Fast Tracks Nektar Therapeutics’ AD Candidate
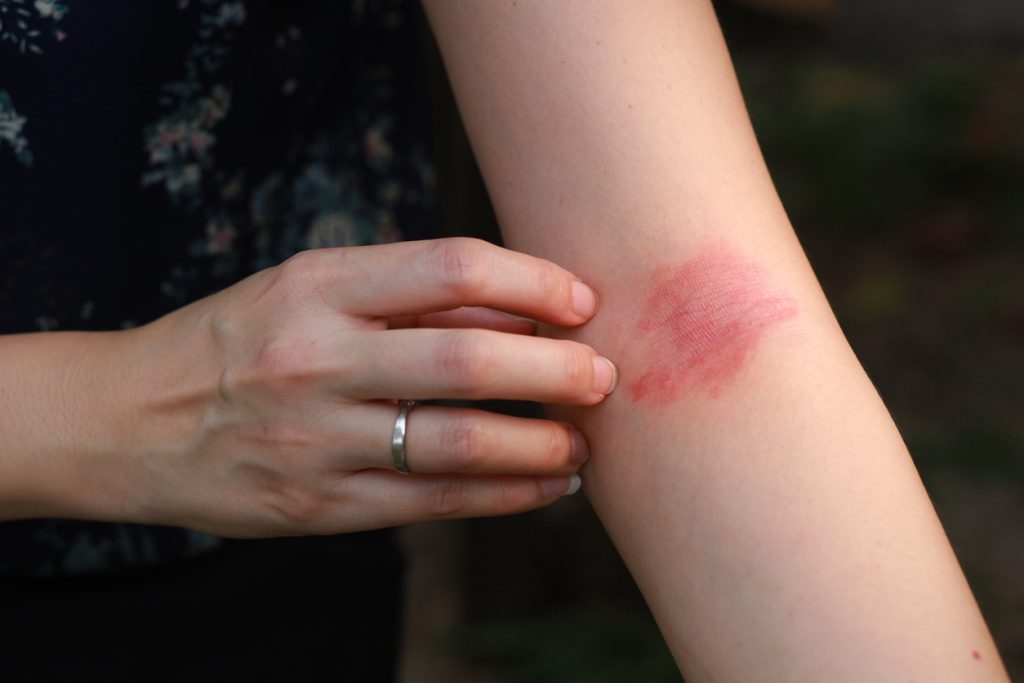
The U.S. Food and Drug Administration (FDA) has granted Fast Track designation for Nektar Therapeutics’ rezpegaldesleukin for the treatment of adult and pediatric patients 12 years of age and older with moderate-to-severe atopic dermatitis (AD) whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Rezpegaldesleukin is an […]
