INmune Bio to Submit FDA BLA for CORDStrom in RDEB
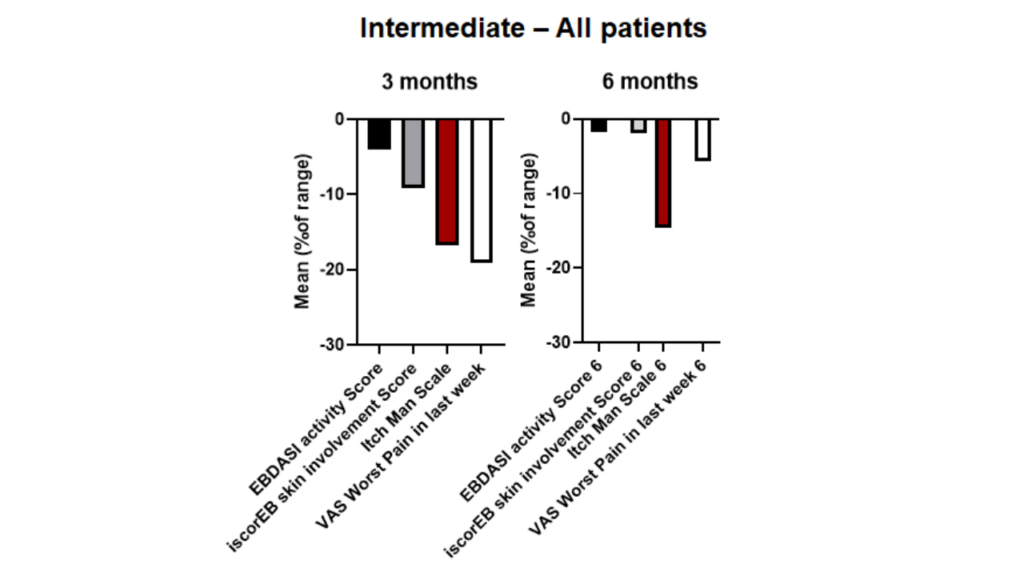
INmune Bio, Inc. is on track to submit a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) and Marketing Authorization Application (MAA) in the UK and EU for CORDStrom in Recessive Dystrophic Epidermolysis Bullosa (RDEB). CORDStrom is a patent-pending, off-the-shelf, advanced mesenchymal stromal cell (MSC) platform developed to treat complex inflammatory […]
