AiViva Biopharma Receives U.S. FDA Clearance to Administer AIV001 in Facial Skin by Intradermal Injection for Nonmelanoma Skin Cancer
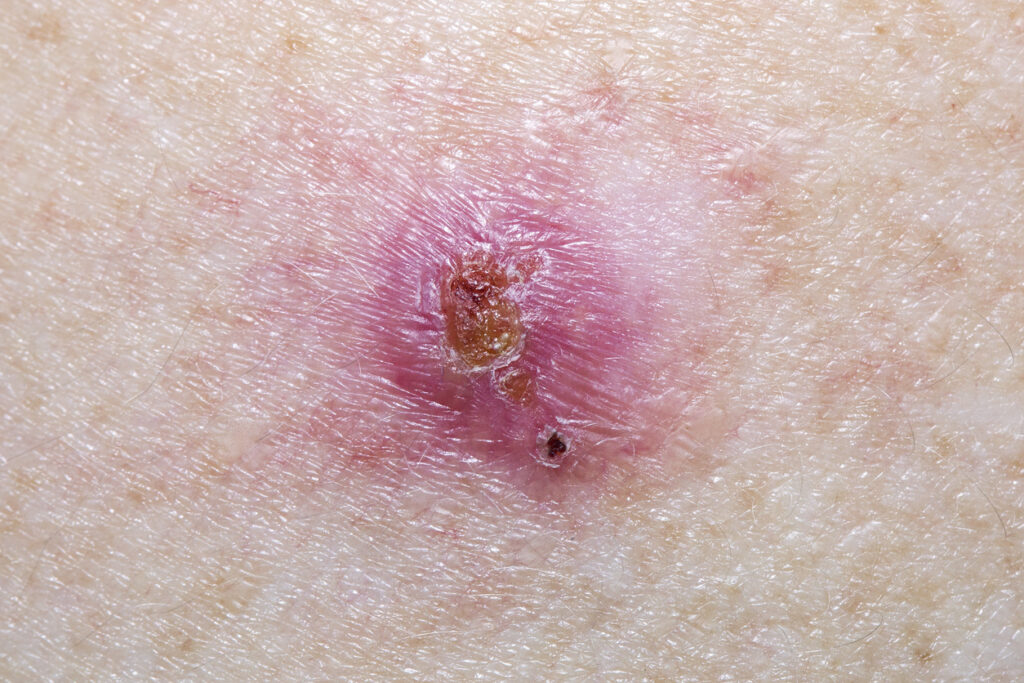
The U.S. Food and Drug Administration (FDA) has given AiViva Biopharma its OK to test AIV001 in facial skin by intradermal injection for nonmelanoma skin cancer. AIV001 is currently in development for evaluation in superficial and nodular superficial basal cell carcinoma. AIV001 is a pan-tyrosine kinase inhibitor in a proprietary formulation designed for prolonged drug […]
