U.S. FDA Grants Orphan Drug Designation for Restem’s ULSC Program for the Treatment of Dermatomyositis and Polymyositis
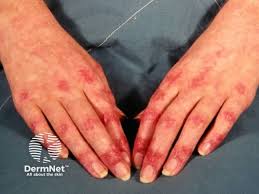
The U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) for Restem’s umbilical cord outer lining stem cells (ULSCs) program for the treatment of dermatomyositis (DM) and polymyositis (PM). Restem’s ULSC therapy has shown promising results in early clinical trials, demonstrating safety, tolerability and initial clinically significant improvements, as well as the […]
