Johnson & Johnson Seeks U.S. FDA Approval for Guselkumab (Tremfya) in Pediatric PsO and jPsA
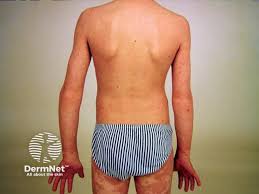
Johnson & Johnson submitted two supplemental Biologics License Applications (sBLAs) to the U.S. Food and Drug Administration (FDA) seeking approval of guselkumab (Tremfya) for the treatment of children 6 and older with moderate-to-severe plaque psoriasis (PsO) and children 5 and up with active juvenile psoriatic arthritis (jPsA). Guselkumab is the first approved monoclonal antibody that […]
