FDA Accepts Dupilumab sBLA for CSU; New PDUFA Date Set
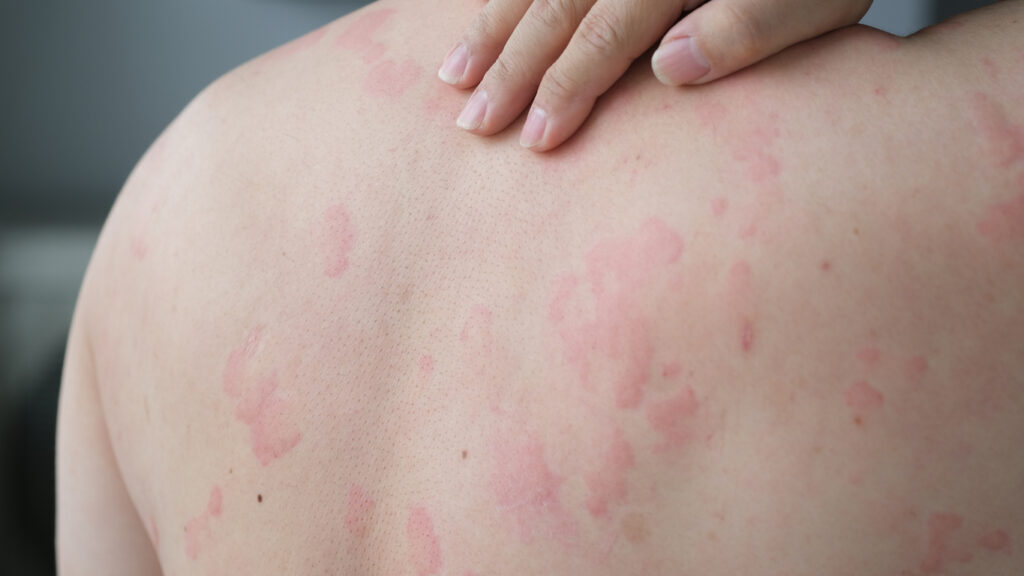
The U.S. Food and Drug Administration (FDA) has accepted for review the resubmission of the supplemental Biologics License Application (sBLA) for dupilumab (Dupixent, Sanofi & Regeneron) to treat adults and pediatric patients aged 12 years and older with chronic spontaneous urticaria (CSU) whose disease is not adequately controlled with H1 antihistamine treatment. The new Prescription […]
