Abeona Therapeutics Resubmits FDA Application for RDEB Gene Therapy
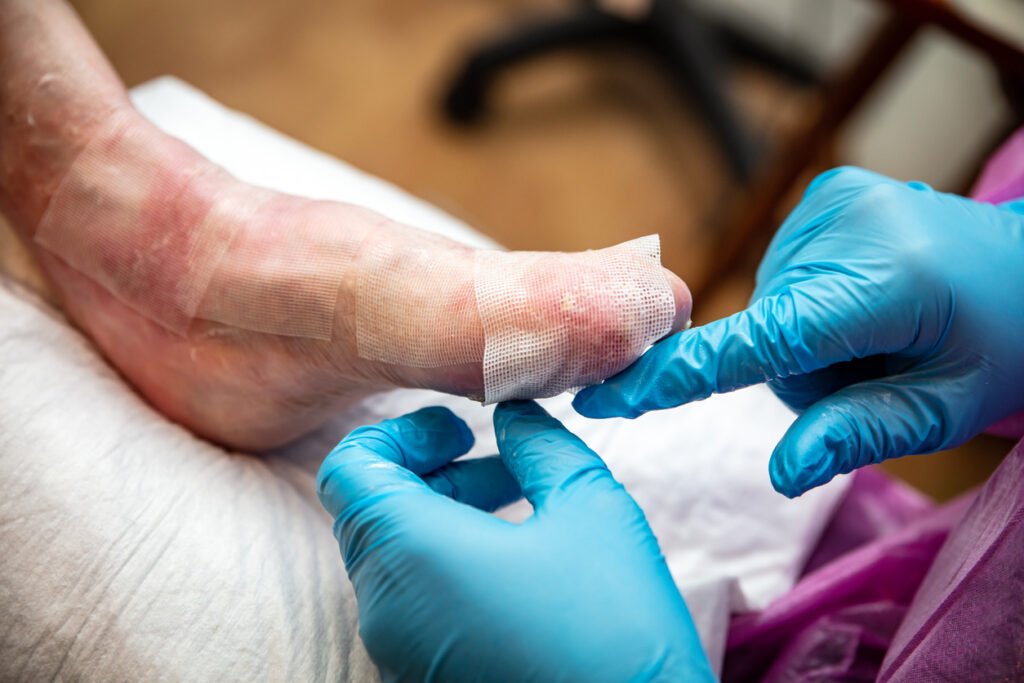
Abeona Therapeutics Inc. has resubmitted its Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for prademagene zamikeracel (pz-cel), its investigational autologous cell-based gene therapy, as a potential new treatment for patients with recessive dystrophic epidermolysis bullosa (RDEB). The BLA resubmission follows the Company’s Type A meeting in August 2024, where Abeona […]
