Lupus Pipeline Watch: Cullinan Therapeutics Submits IND to FDA for CLN-978
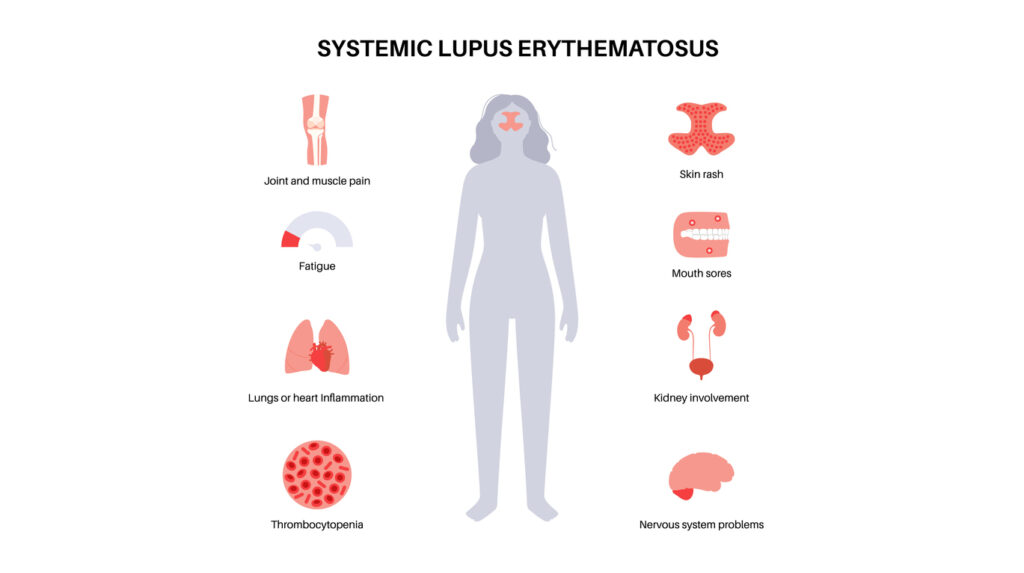
Cullinan Therapeutics, Inc. has submitted an Investigational New Drug (IND) application to the U.S. Food and Drug Administration (FDA) to evaluate its CD19xCD3 bispecific T cell engager, CLN-978, for the treatment of systemic lupus erythematosus (SLE). CLN-978 is a novel, highly potent, half-life extended CD19xCD3 bispecific T cell engager construct. CLN-978 potently triggers redirected lysis […]
