FDA Approves Alembic’s Generic Scalp Psoriasis Treatment
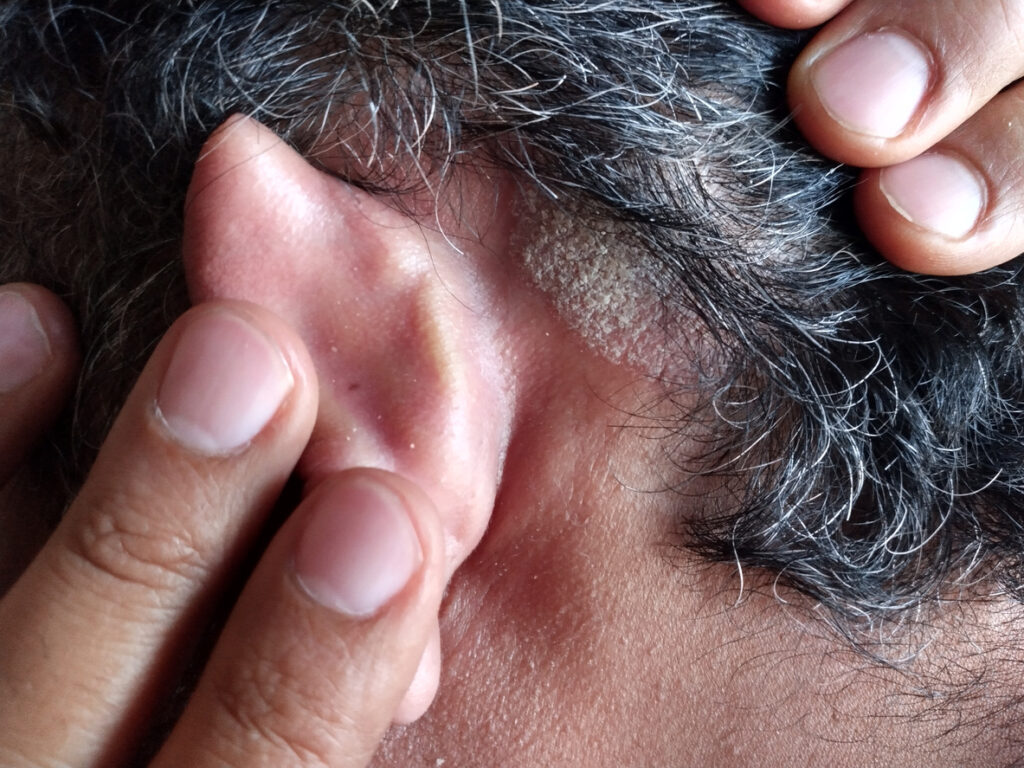
The U.S. Food and Drug Administration (FDA) has approved Alembic Pharmaceuticals’ generic Betamethasone Valerate Foam, 0.12% for moderate-to-severe psoriasis of the scalp The Abbreviated New Drug Application (ANDA) is therapeutically equivalent to the reference listed drug product Luxiq Foam, 0.12% from Norvium Bioscience, LLC. Betamethasone valerate foam, 0.12% is a medium potency topical corticosteroid indicated […]
