FDA Approves IND Application for Azitra, Inc.’s ATR-04 in EGFRI-associated Skin Rashes
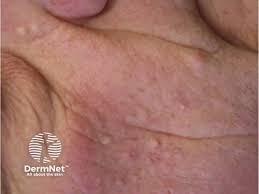
The U.S. Food and Drug Administration (FDA) has cleared Azitra, Inc.’s investigational new drug (IND) application for a first-in-human Phase 1/2 clinical study of ATR-04 for moderate to severe epidermal growth factor receptor inhibitor (EGFRI)-associated dermal toxicity. ATR-04 is a live biotherapeutic product candidate including an isolated, naturally derived Staphylococcus epidermidis strain that was engineered to be […]
