European Commission Approves 320mg Syringes and Pens of UCB’s Bimekizumab
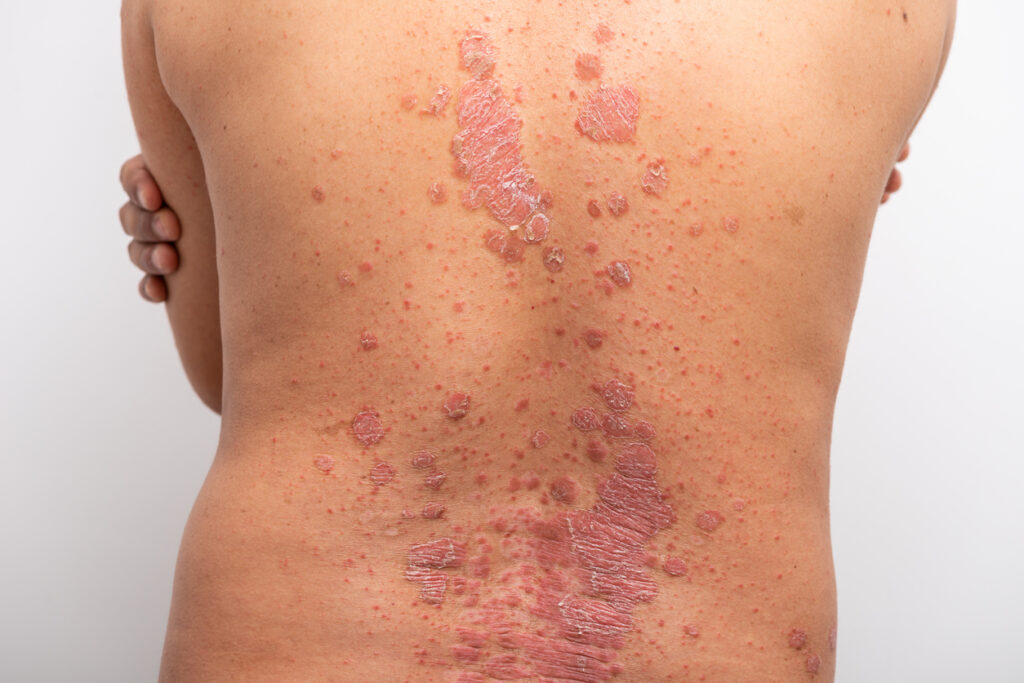
The European Commission (EC) has granted marketing authorization for two 320mg device presentations of UCB’s bimekizumab (Bimzelx, UCB). The pre-filled syringe and pre-filled pen each contain 320 mg of bimekizumab in a volume of 2mL and provide alternatives to the currently available 160 mg in a volume of 1 mL injection options. UCB developed the […]
