Arcutis Submits sNDA to FDA for ZORYVE Foam 0.3% for Scalp and Body Psoriasis in Adults and Adolescents Ages 12 and Older
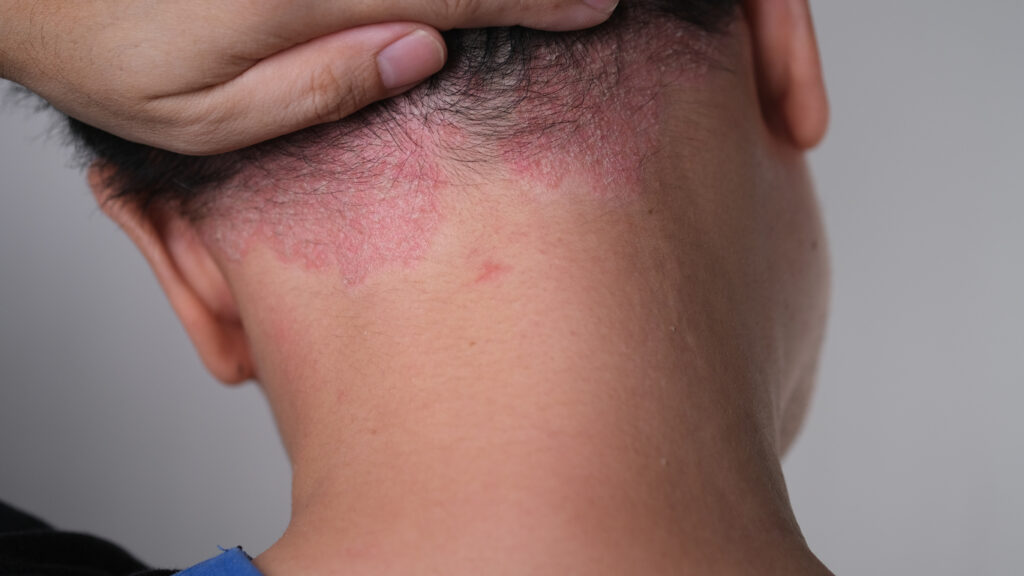
Arcutis Biotherapeutics submitted a supplemental new drug application (sNDA) to the U.S. Food and Drug Administration (FDA) for roflumilast foam 0.3% (ZORYVE), a once-daily, next generation phosphodiesterase-4 (PDE4) inhibitor, for the treatment of adults and adolescents ages 12 and older with scalp and body psoriasis. In clinical trials, roflumilast foam 0.3% was effective and well-tolerated, […]
