FDA Nod for PYZCHIVA (ustekinumab-ttwe), Samsung Bioepis’ Stelara Biosimilar
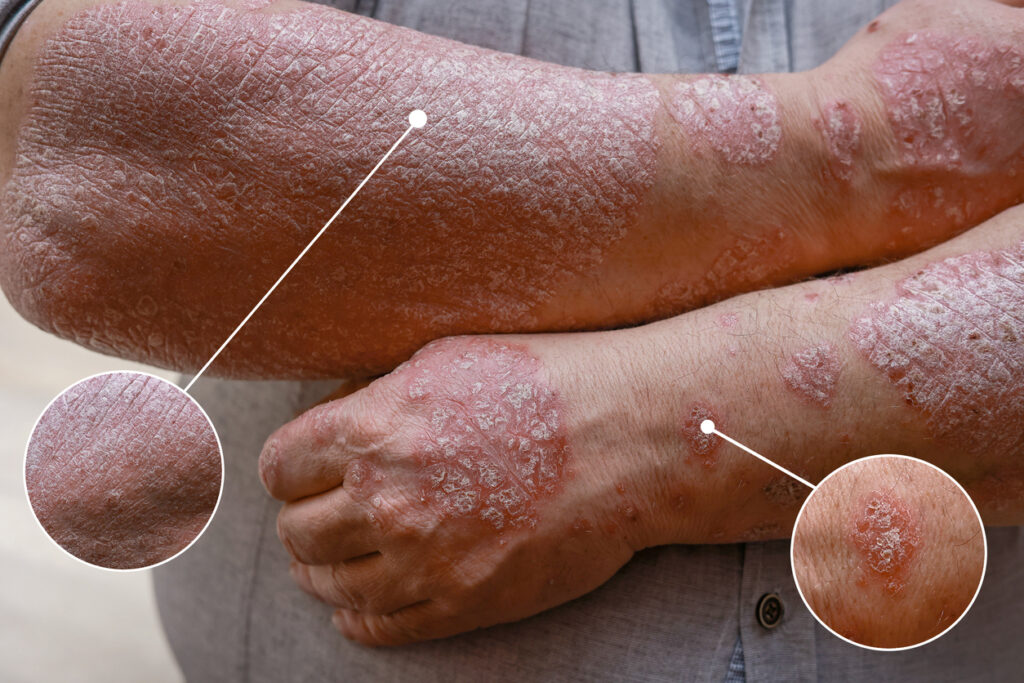
The U.S. Food and Drug Administration (FDA) has approved the Biologics License Application (BLA) for PYZCHIVA (ustekinumab-ttwe) subcutaneous injection and intravenous infusion as a biosimilar to Stelara (ustekinumab). PYZCHIVA has been approved for the treatment of moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderately to severely […]
