FDA Nod for Avita’s RECELL GO System for Burns and Full-thickness Skin Defects
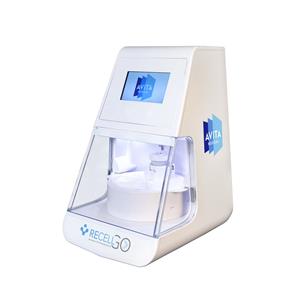
The U.S. Food and Drug Administration (FDA) has approved AVITA Medical, Inc.’s premarket approval (PMA) supplement for RECELL GO System, a next-generation autologous cell harvesting device that harnesses the regenerative properties of a patient’s skin to treat thermal burn wounds and full-thickness skin defects. The device uses a small sample of the patient’s skin to […]
