New FDA Nod: UltraClear Now Approved for Treating Benign Pigmented Lesions and Vascular Dyschromia
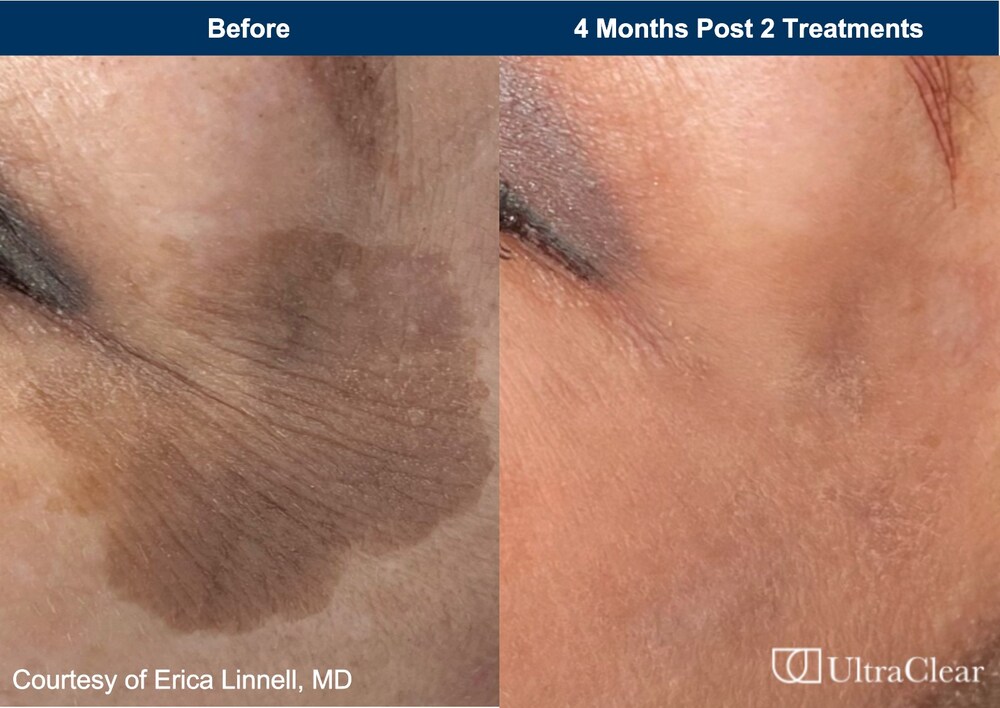
The U.S. Food and Drug Administration (FDA) has cleared Acclaro Medical’s UltraClear cold ablative fractional 2910 nm fiber laser for the treatment of benign pigmented lesions and vascular dyschromia. The laser is already cleared for general skin resurfacing procedures and treating fine lines and wrinkles, benign age spots, scars, and skin tone. “Any change in skin […]
