Major Step Forward: FDA Clears IND Application for Vyne’s BD2-Selective BET Inhibitor
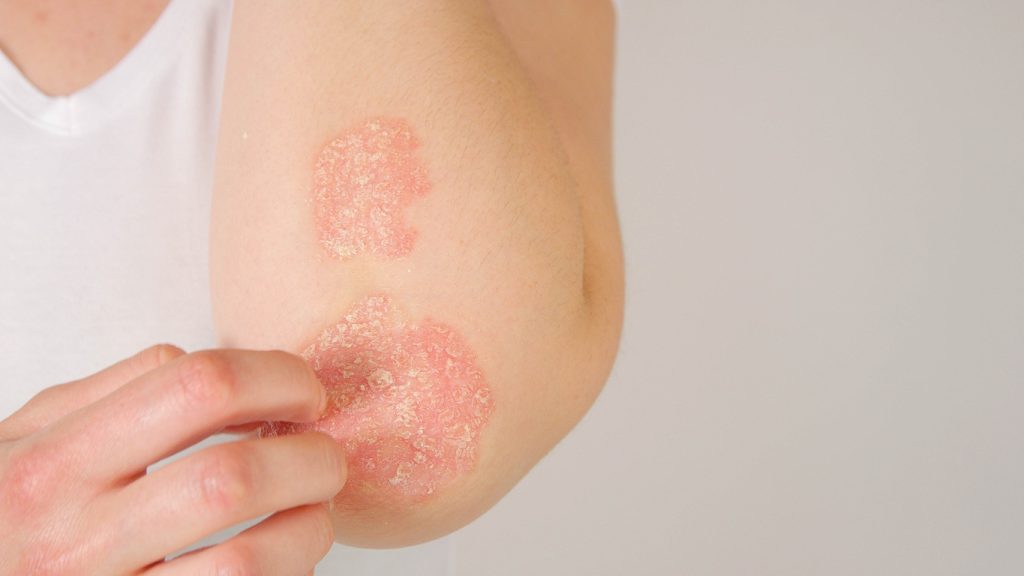
The U.S. Food and Drug Administration has cleared the Investigational New Drug (IND) application for VYN202, an oral small molecule BD2-selective BET inhibitor that may play a role in treating immuno-inflammatory disease, Vyne Therapeutics reports. The company now plans to initiate a first-in-human Phase 1a single ascending dose/multiple ascending dose (SAD/MAD) trial in healthy volunteers […]
