New Data Supports the Role of Castle’s DecisionDx-Melanoma GEP Test for Assessing the Need for SLN Biopsies
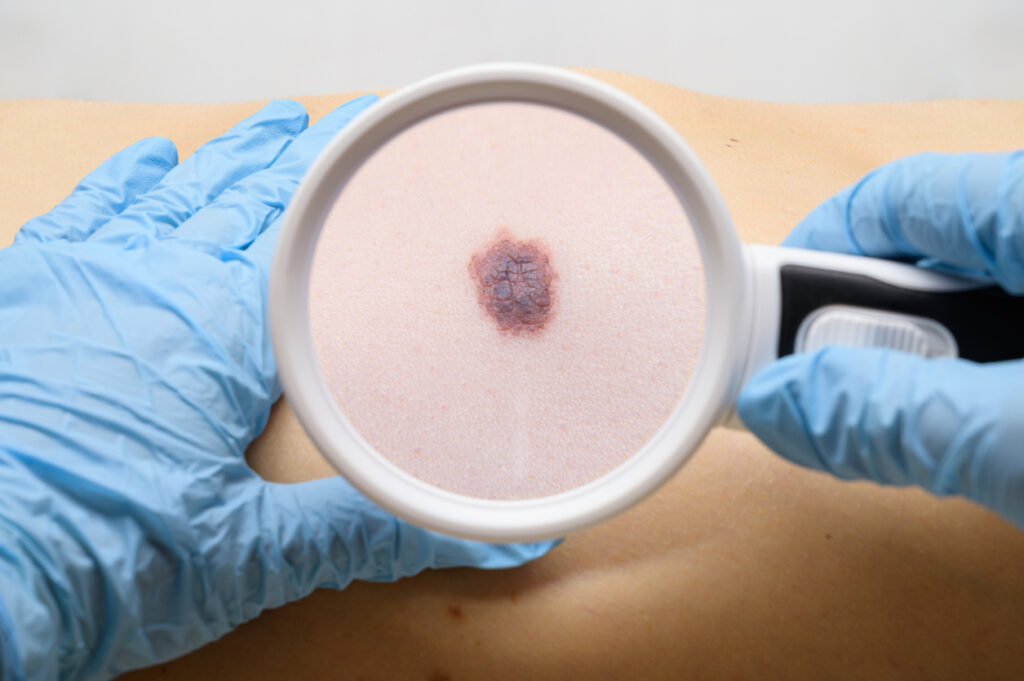
Castle Biosciences, Inc.’s DecisionDx-Melanoma test can reduce unnecessary sentinel lymph node (SLN) procedures by about 25%, lowering the costs and risks of complications that can accompany these biopsies, according to new research presented at the Society of Surgical Oncology 2024 Annual Meeting in Atlanta. DecisionDx-Melanoma has been designed and validated to inform two clinical questions […]
