HS Pipeline Watch: MoonLake Immunotherapeutics’ Sonelokimab Heads to Phase 3
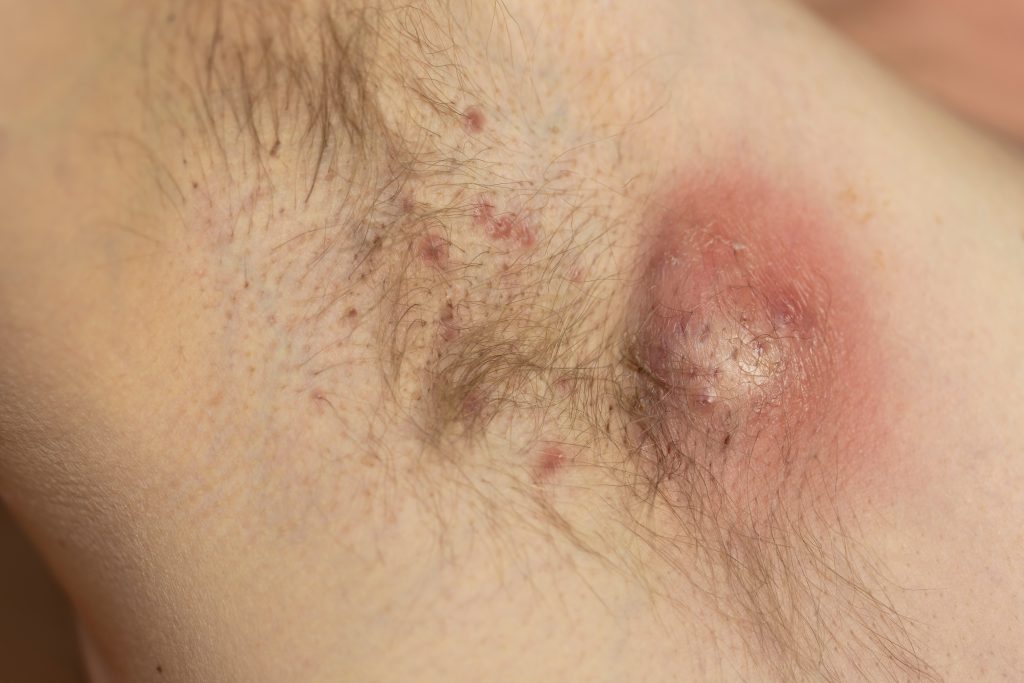
The U.S. Food and Drug Administration (FDA) and E.U. European Medicines Agency (EMA) have greenlighted MoonLake Immunotherapeutics’ Phase 3 Program for the nanobody sonelokimab (SLK) in hidradenitis suppurativa (HS). Sonelokimab is a novel investigational nanobody for the treatment of inflammatory disease. Sonelokimab inhibits IL-17A and IL-17F by inhibiting the IL-17A/A, IL-17A/F, and IL-17F/F dimers that drive inflammation. Sonelokimab is […]
