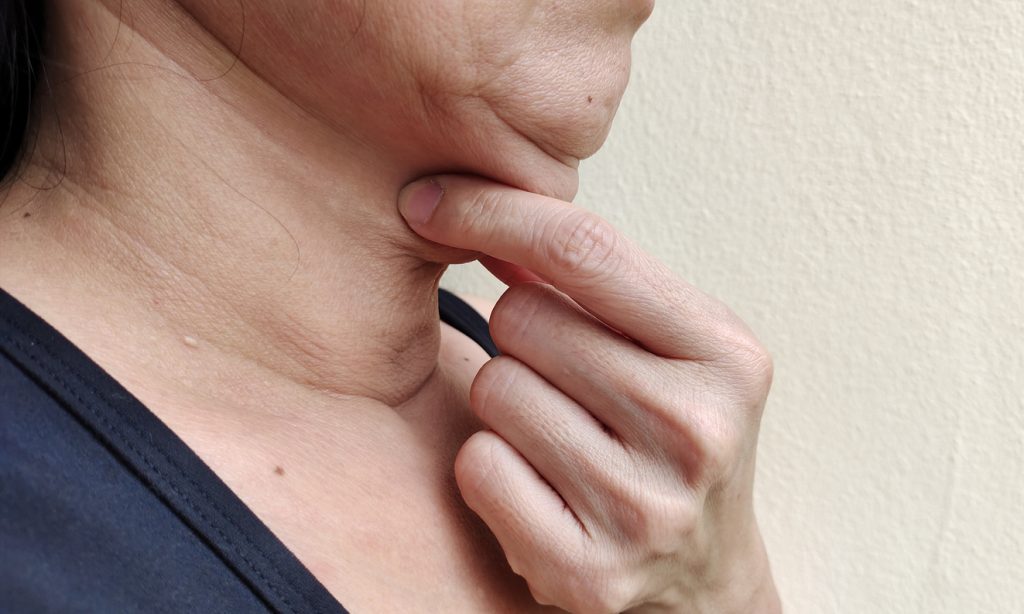
Injectable 10XB101 may beat the competition when it comes to submental body contouring in terms of efficacy and tolerability, according to research presented at the 2024 American Academy of Dermatology (AAD) Annual Meeting in San Diego, CA.
10xBio’s 10XB101 is proprietary formulation of polidocanol, which is approved in the U.S. for vein sclerotherapy.
In this randomized study with three active treatment groups, patients received multiple injections of 10XB101 in a predefined pattern, with up to six treatments spaced four weeks apart. Efficacy was assessed by clinician- and patient-based assessments (CSFS and PSFS) using a submental fat rating scale by comparing baseline to outcomes four weeks after their last treatment.
“The results of this study clearly demonstrated far superior efficacy and tolerability for 10XB101 than the currently marketed body contouring drug, deoxycholate (Kybella, Allergan Aesthetics), which I have more than 10 years of experience with,” says Mitchel P. Goldman, MD, a dermatologic surgeon and medical director of Cosmetic Laser Dermatology in San Diego, CA, in a news release. “10XB101 has the appropriate product-market fit to capture the tremendous opportunity for submental contouring. In addition, its attributes hold promise for potential expansion to other body contouring applications, such as treatment of the abdomen and flanks.”
Key study findings include:
- In the intent-to-treat (ITT) population, more than 50% of patients in the two higher dose groups achieved a concordant Grade 2 or more improvement in both their CSFS and PSFS scores, the FDA designated primary endpoint, compared to 0% in the placebo group.
- In the completer population (e.g. patients receiving at least four doses of 10XB101), about 80% of patients in the two higher dose active groups demonstrated concordant Grade 2 or more improvement in both their CSFS and PSFS scores, compared to 0% in the placebo group.
- Onset of effect was rapid in the higher dose groups with more than 65% of ITT and more than 84% of completer populations achieving a minimum of a Grade 1 improvement or greater after their initial two treatment sessions.
- Local injection site reactions were measured using a four-point scale ranging from absent to severe and included bruising, edema, pain and erythema at each visit during the trial. About 98% of injection site reactions were measured as zero (absent) or one (mild).
The results compare favorably to deoxycholate, with up to four times as many patients achieving concordant Grade 2 or more improvement on the CSFS/PSFS. With 10XB101, the frequency and severity of injection site reactions are meaningfully lower than with its counterpart.